Image Credit: screenshot/YouTube
FDA Panel Rubber-Stamps Covid Injection For Kids 5-11: ‘Never Gonna Learn How Safe the Vaccine is Until We Start Giving It’
“That’s the way it goes,” says voting member, “And I think we should vote to approve it.”
A Food and Drug Administration (FDA) advisory committee recommended on Tuesday the COVID-19 injection for children 5 to 11 despite admitting not knowing the long-term risks.
The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) voted in panel 17-0 to approve a lower dose of the Pfizer’s COVID injection for about 28 million American children through emergency use authorization.
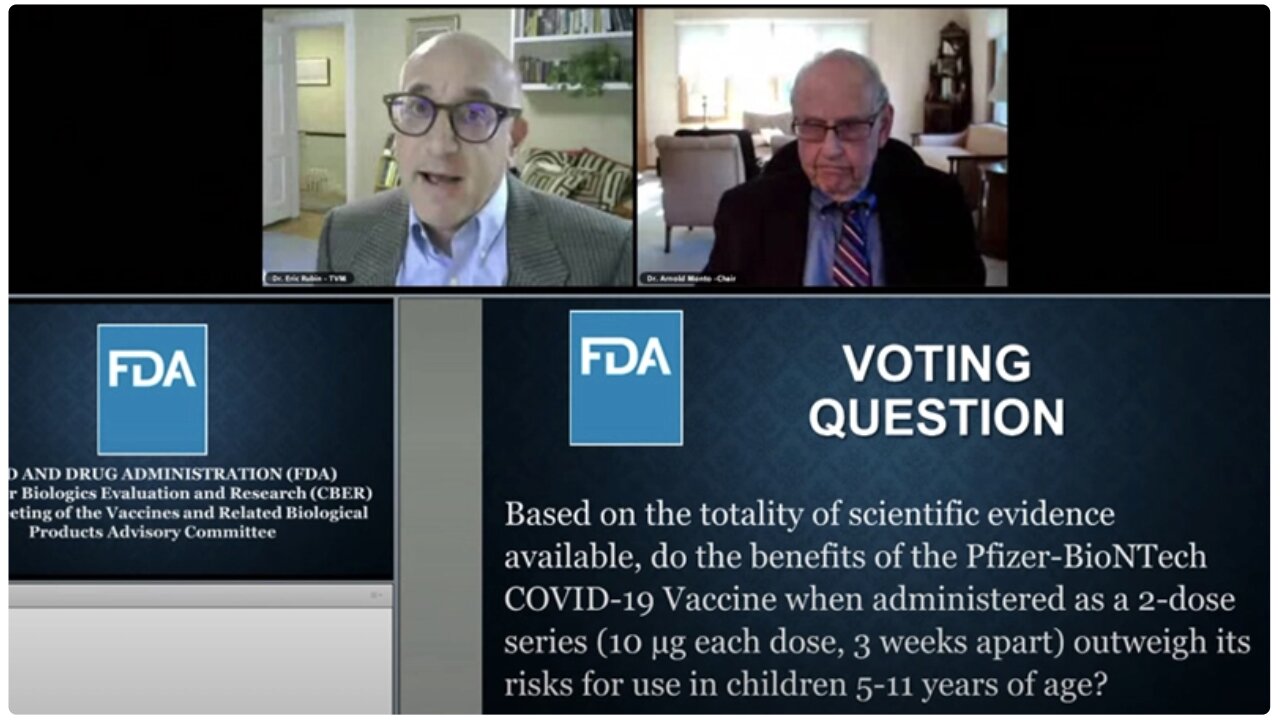
The panel convened to answer the following question: “Based on the totality of scientific evidence available, do the benefits of the Pfizer-BioNTech COVID-19 Vaccine when administered as a 2-dose series outweigh its risks for use in children 5-11 years of age?”
Dr. Eric Rubin, one of the voting members, admitted nobody knows the long-term effects of the Covid injection in children, which is why it should be approved.
“We’re never gonna learn about how safe the vaccine is until we start giving it. That’s the way it goes,” he said. “And I think we should vote to approve it.”
In other words, they need to approve it to experiment on America’s children to learn if it’s safe for children.
The FDA briefing document the panel used to come to its conclusion outright admitted it has no available data for myocarditis cases in children 5 to 11, so it assumes it would be at about the same notable myocarditis rate as children 12-15.
“For this analysis the estimate for ages 12-15 years is applied to ages 5-11 years because vaccine associated myocarditis/pericarditis data is not available for this age group,” the document admits.
NIAID Director Anthony Fauci said Sunday that the COVID shot will likely be quickly approved if the FDA panel signed off on it.
“If all goes well, and we get the regulatory approval and the recommendation from the CDC, it’s entirely possible, if not very likely, that vaccines will be available for children from 5 to 11 within the first week or two of November,” Fauci said on ABC’s “This Week.”
Watch the full VRBPAC meeting:
Twitter: @WhiteIsTheFury
Gab: @WhiteIsTheFury
Minds: @WhiteIsTheFury
Gettr: @WhiteIsTheFury
Learn the True History of Fauci’s Horrific Medical Experiments on Children and Dogs

